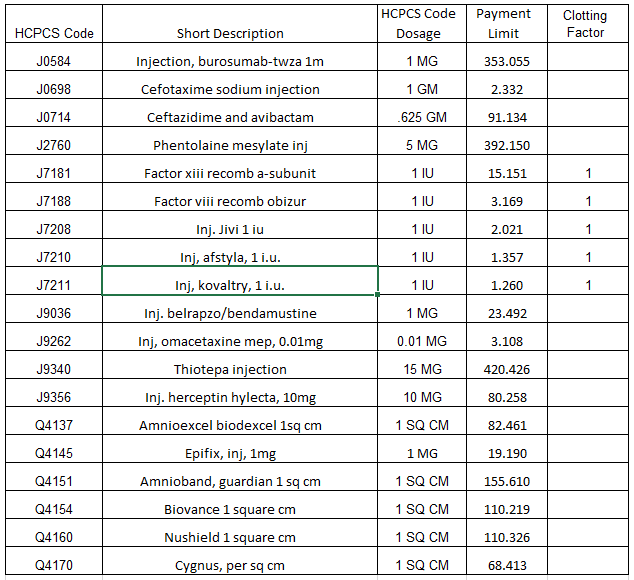
Review the rationale behind the legislation and learn the reason for the update. The Centers for Medicare & Medicaid Services (CMS) recently released its seventh annual update to the Drug Spending Dashboards to include data for 2021 as the agency continues to implement the Inflation Reduction Act. What Is the Inflation Reduction Act? The Inflation […]
The post CMS Updates Drug Spending Dashboards appeared first on AAPC Knowledge Center.